The Cansolv System Process: A new paradigm for SO2 recovery and recycle
By John N. Sarlis and Patrick M. Ravary
Cansolv Technologies Inc.
8475, avenue Christophe-Colomb, Suite 2000
Montreal, Quebec, H2M 2N9 Canada
http://www.cansolv.com
The Cansolv System is an aqueous amine-based regenerative gas desulfurization process that promises SO2 removal down to a few ppm. It is flexible, robust, easy to operate ,and quickly responsive to changes in gas feed conditions. Potential uses include sulfuric acid plants, spent acid recovery plants, sulfide ore smelters, sulfur recovery units, flue gas desulfurization units, pulp mills, and SO2 production, storage, and transportation. The first of three articles reviews the process itself.
Contents
Cansolv system technology
Process description
Equipment mechanical design
Technology development status
Introduction
Environmental regulations are trending towards stricter values of allowable emissions for all pollutants, from all sources, in all jurisdictions. Often, regulatory authorities require use of best available commercial technology (BACT), and more recently, maximum achievable commercial technology (MACT). This increases capital and operating costs. The Cansolv System Process introduces a new paradigm for SO2 recovery and recycling by simultaneously reducing emissions and cost.
The dominant desulfurization technology today is limestone or lime-based scrubbing in various forms. While in general reliable and, in some forms, capable of high SO2 removal efficiency, both produce large quantities of low-value waste products. They are relatively expensive to build and operate, and difficult to retrofit in constrained sites due to the large equipment size. With increasing concern over the cost and availability of landfill sites and public demand for resource recovery and recycling, recovery-type SO2 removal processes are becoming increasingly more desirable.
Recovery processes have been used by some utilities and industrial companies to remove SO2 and to produce wallboard-grade gypsum or other useful products. For many utilities and industries (such as oil refining, natural gas processing, smelting or pulp and paper), the preferred byproducts are sulfur dioxide, elemental sulfur or sulfuric acid. Regenerable processes based on sodium sulfite and MgO have addressed this need, but have gained limited commercial acceptance due to high costs.
The Cansolv System is an aqueous amine-based, regenerative gas desulfurization process capable of removing SO2 down to a few ppm, if desired, from most stationary sources. The process can be applied to feed gases with <0.1 to 100% SO2. It is flexible, robust, easy to operate and quickly responsive to changes in gas feed conditions.
Cansolv represents an effective response to domestic and international regulation-driven market needs in a manner that vastly improves upon existing desulfurization technologies in terms of physical dimensions, capital and operating costs, and environmental impact. In many cases, the Cansolv System can be integrated to an existing plant flowsheet to improve profitability of the process.
The Cansolv System can be applied to sulfuric acid, spent acid recovery plants, sulfide ore smelters, sulfur recovery units, SO2 production and its safe storage and transportation, flue gas desulfurization units and pulp mills.
Cansolv system technology (Back to Top)
Process chemistry
Due to technical simplicity, economic advantage and lack of waste by-product, regenerable SO2 absorption with homogeneous aqueous absorbents is generally preferred over once-through processes. In water solution, dissolved SO2 undergoes reversible hydration and ionization according to the following equations:
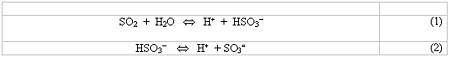
Reactions (1) and (2) are half completed at pH values of 1.81 and 6.91, respectively, at 18°C 2. The amount of SO2 dissolved can be increased by adding a buffer, such as an amine, to the water. The buffer drives the above equilibria to the right by reacting with the hydrogen ions to form ammonium salts:

In order for the process to be regenerable, the buffering agent should operate in a pH region sufficiently low so as to present a desirable value of SO2 vapor pressure over the solution at the regeneration temperature. Steam stripping of the vapor phase SO2 in a multiple stage column will then reverse reactions (1) - (3), regenerating the absorbent.
Sulfite anions, added to water as a salt such as sodium sulfite, can also be utilized as a buffer:

This is the basis of the Wellman-Lord process. However, since the sulfite anion is a fairly strong base (pKa = 6.91), it buffers at too alkaline a pH. This has the effect of making the regeneration of the solution more difficult, resulting in increased steam usage and/or incomplete stripping.
The Cansolv System process is based on a unique class of diamine absorbents that optimally balance the ability to absorb and regenerate sulfur dioxide. One of the amine functionalities of the absorbent is so strongly basic that it is not thermally regenerable under the Cansolv System process conditions. So, once reacted into a salt by SO2 or any stronger acid, this strongly basic amine functionality will remain permanently as a salt while in process. This is illustrated in Eq. 5, for the case of virgin diamine reacting for the first time in process with an acid HX, where X- is any anion of a relatively strong acid, such as Cl - , NO3- etc. A strong dibasic acid such as sulfuric would protonate two amines and yield SO4= as the anion X-.
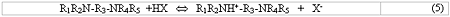
The monoprotonated amine on the right hand side of Eq. 5 is the in-process lean amine which is used to scrub SO2 . Because it is a salt, it is totally non-volatile and since it is non-heat regenerable, it will stay in salt form throughout the process.
The second amine functionality (the ‘sorbing nitrogen') is less basic and it buffers in the desired range for regenerability of SO2, which in practice is about pH 4 for the rich amine and pH 6 for the lean. This buffering range provides the proper balance of absorption and regenerability and is the essence of the Cansolv technology. This reaction is shown in Eq. 6.
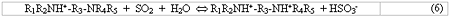
In Eq. 6, the anion X- is not shown since it does not participate in the SO2 reaction with the sorbing nitrogen. The nature of X- can affect the functioning of the process: if it is sulfite (SO3=), it can contribute to SO2 scrubbing according to Eq. 4. However, if it is X-, the anion of a strong acid, and is allowed to accumulate to more than 1 equivalent per mole of amine, it will neutralize the sorbing nitrogen and thereby decrease the SO2 scrubbing capacity of the solvent. Thus, the level of these ‘heat stable salts' (HSS), is kept below 1 equivalent per mole by a slipstream electrodialysis purification unit which replaces non-regenerable anions such as sulfate by sulfite or bisulfite.
The diamine absorbents are described in the basic process patent, US Patent 5,019,361. Normally, a 25-30% solution of the amine in water is utilized in the process.
These absorbents provide significant advantages:
Compared to alkaline salt absorbents such as sodium sulfite or sodium phosphates, the amine absorbents of the Cansolv technology have the very significant advantage of easy HSS removal by electrodialysis as described in US Patent 5,292,407.
Other advantages of the Cansolv absorbent are:
The absorption of SO2 by the absorbent is gas side mass transfer limited, since the reactions of SO2 in solution are for all practical purposes instantaneous. This minimizes scrubber cost and makes possible the use of innovative mass transfer devices.
The absorbent is highly selective for sulfur dioxide over carbon dioxide - by a factor of about 50,000. Acids stronger than SO2, such as sulfuric and hydrochloric acids, are also absorbed effectively. However, since they are not heat regenerable, they are removed from the solvent by electrodialysis in a slipstream solvent purification unit. The absorbed SO2 is completely recovered as SO2 product. There is negligible oxidation and loss to SO3.
Process description (Back to Top)
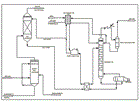
The generic Cansolv System scrubbing process was successfully piloted during a 9-month gas flue desulfurization trial in 1991 at the Suncor Oil Sands plant in Fort McMurray, Alberta(5). The process as shown in Figure 1 has four main components, excluding conventional equipment such as waste heat boilers, byproduct SO2 conversion processes, and wastewater treatment systems.
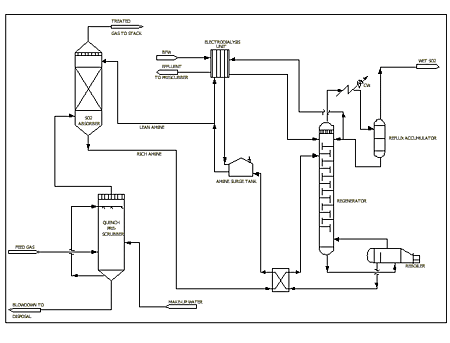
Prescrubber. The prescrubber contacts the oxidized feed gas with recirculated water in a spray tower. This cools and saturates the feed gas and removes a large fraction of the particulates, depending on their size. Strong acids such as sulfuric and hydrochloric acid are also scrubbed, decreasing the duty on the HSS removal unit. In cases where the adiabatic saturation temperature of the feed gas is high (such as in a sulfur recovery unit tail gas cleanup unit application), the prescrubber is also used as a direct cooler by adding a heat exchanger to the water circulation loop (analogous to a Quench Column). The level of dissolved acids in the prescrubber water is controlled by blowdown, neutralization, and discharge into a wastewater treatment system. Suspended solids are controlled by settling.
When treating strong acid and particulate free gas, such as sulfuric acid plant tail gas, the primary function of the prescrubber would be to saturate the feed gas prior to SO2 absorption. Saturation of the feed gas will occur in the absorber in any case, making a separate prescrubber optional. In such a case, continuous water addition to the amine absorbent would be used to maintain the water balance of the amine solution.
Absorber. The absorber is a mass transfer device for contacting the absorbent with the feed gas. Since the Cansolv System absorbent reacts reversibly with SO2, multistage countercurrent contacting must be used to achieve maximum loading of the acid gas in the rich absorbent. Any conventional absorber type may be used, such as a packed or trayed tower. The selection of absorber type is based on normal engineering and economic considerations.
Regenerator. The rich SO2 laden absorbent from the absorber is pumped to the regenerator via a lean/rich heat exchanger. The regenerator is normally a trayed or packed tower with a steam heated reboiler. As the absorbent flows down the column, the SO2 is stripped from the liquid and carried overhead into a reflux condenser, where most of the steam condenses and is returned to the top of the regenerator as reflux. The gaseous, water saturated sulfur dioxide leaves the regenerator via a blower or compressor. The lean amine leaves the reboiler and is pumped back to the absorber via the lean/rich heat exchanger, a trim amine cooler, and a surge tank.
Amine purification unit. A slipstream of effectively about 0.1-0.3% of lean amine flow is fed to the electrodialysis HSS removal unit. There, heat stable anions like sulfate are replaced by regenerable sulfite anions sourced from the stripper reflux. The heat stable anion containing waste stream is pumped into the prescrubber water loop.
Equipment mechanical design (Back to Top)
Since the Cansolv System SO2 absorption process is very similar to the well known amine treating processes for removal of H2S and CO2 from refinery streams and natural gas, the same engineering methods, equipment selection and process control choices are generally applicable to both systems. Materials of construction are adjusted to handle the lower pH values resulting from the higher acidity of SO2 compared to H2S and CO2.
Because SO2-containing feed gas streams often contain stronger acids such as sulfuric acid, the rate of heat stable salt accumulation is higher than in conventional amine treaters. Disproportionation of sulfite may also contribute to HSS accumulation. Therefore, in contrast to conventional amine treaters, an HSS removal unit is included in the process equipment.
Technology development status (Back to Top)
Development of the Cansolv System SO2 scrubbing process was begun by Union Carbide Canada Ltd. in 1988. Studies of possible novel absorbents led to the issue of U.S. Patent 5,019,361 in 1991 for the basic process. The required physical property and safety data were generated to support engineering of a large, versatile pilot plant, which was operated for 9 months at the Suncor Inc. Tar Sands Plant in Fort McMurray, AB. The unit operated from February 28 to November 28, 1991, scrubbing 3600 scfm of flue gas from the site's utility boilers. The fuel in the boilers, petroleum coke produced in the tar upgrading process, had a sulfur content of 7%. On many occasions in the course of performing the statistically designed experiments, SO2 in the treated gas was <15 ppmv.
Results of the test program exceeded expectations and demonstrated the Cansolv System process to be robust, effective and easy to operate. A statistical process model was developed based on the test data. Subsequently, a rigorous thermodynamic model was generated on the ASPEN Plus platform. The two models are in good agreement.
In 1992, Union Carbide received an award under the U.S. Department of Energy's Clean Coal Program to demonstrate the process by scrubbing flue gas from a coal fired boiler at Alcoa's 75 MW Warrick Generating Station in Indiana. Further engineering and laboratory studies were conducted to support this project and a Definition of Technology was compiled. At this point, Union Carbide cancelled the Cansolv System program due to a corporate strategic decision to concentrate on core competencies in the petrochemical area. Key technical personnel involved in the process development purchased the technology from Union Carbide in 1997 in order to commercialize it.
Heat stable salt removal from the process absorbent is effected by a metathesis electrodialysis (ED) process under U.S. Patent 5,292,407. Electrodialysis of this form, sometimes called double decomposition electrodialysis, is utilized commercially in other applications, while simple two-compartment ED heat stable salt removal from refinery amine treater solvents is practiced commercially (6). The Superclean Cansolv System process flowsheet modification has been patented (7). This modification permits removal of SO2 in the treated gas to essentially zero.
A patent application has been filed for the SO2SAFE sulfur dioxide storage technology, an inherently safer method compared to liquid SO2 storage. The sulfur dioxide is held in solution at atmospheric pressure and regenerated by steam stripping on demand. In the event of a leak or spill of the loaded solution, only a relatively small amount of SO2 is released, greatly reducing the hazard. This process can be used as buffer storage of SO2 in order to decouple process units, or to safely transport SO2 and regenerate it on demand.
Another Cansolv Technologies Inc. process, "Cansolv SRU," is discussed elsewhere (8). This application of Cansolv System SO2 scrubbing is covered by patents (9).
Cansolv Technologies Inc., along with their engineering company partners Black & Veatch and Simon-Carves Fenco Inc., are actively pursuing applications to various industries. During these efforts, these partners are critically challenging the process technology and current process designs to ensure successful commercial application. The companies are currently bidding for the first applications of the technology.
The next installment of this article will discuss specific markets and applications for the technology.
Edited by Alan S. Brown
Managing Editor, Chemical Online
