Removal of Weakly-Ionized Species by EDI
Electrodeionization (EDI), a membrane process used in the production of ultrapure water, has grown rapidly over the last several years [1][2]. In many cases EDI has been the chosen process over conventional batch-type ion-exchange processes.
 There are some obvious reasons for this, such as the environmental benefit of not needing hazardous regenerant chemicals and the inherent superiority of a continuous process over a batch process. There are also the benefits of high product water quality from EDI, especially when compared to other membrane processes [3][4]. Finally, there are the economic benefits of EDI which make this process a real success.
There are some obvious reasons for this, such as the environmental benefit of not needing hazardous regenerant chemicals and the inherent superiority of a continuous process over a batch process. There are also the benefits of high product water quality from EDI, especially when compared to other membrane processes [3][4]. Finally, there are the economic benefits of EDI which make this process a real success.
Another, little documented reason why EDI has succeeded over other processes is the ability for it to continuously remove weakly-ionized species. This is a very important aspect of EDI performance, and in many ways, is what separates this process from other demineralization processes. Normally, weakly-ionized species, such as carbon dioxide, boron, and ammonia are difficult to remove via such membrane processes as reverse osmosis (RO) and electrodialysis reversal (EDR) [5][6][7]. Whereas the key gauge of an RO unit's performance is its degree of silica removal, ion-exchange performance is based on how well weakly-ionized species are removed. EDI offers the benefit of continuous removal of these species to a very high degree. This paper focuses on how the EDI process successfully removes various weakly-ionized species including silica, CO2, boron, and ammonia.
EDI Process
EDI is an electrically-driven membranes process. EDI combines ion-exchange resins, ion-exchange membranes, and a DC electrical field. Figure 1 illustrates the basic flow scheme of the EDI process. In EDI, ionized species are removed much like conventional electrodialysis (ED), with the rate of ion removal greatly increased by the presence of the ion-exchange resins in the diluting cell.
These ion-exchange resins facilitate mass transfer of weakly-ionized species mainly due to the phenomena known as "water splitting". In the diluting cell, the DC electrical field splits water at the surface of the ion-exchange beads, producing hydrogen and hydroxyl ions which act as continuous regenerants of the ion-exchange resins. This allows a portion of the resins in the EDI to always be in the fully-regenerated state. In turn, the fully-regenerated resins are able to ionize weakly-ionized species. Once ionized, these species are quickly removed under the influence of the DC electrical field.
In effect, ionized species are removed in one area of an EDI stack, and weakly-ionized species are removed in a second area. Figure 1 roughly depicts how different ions are removed as water travels through the EDI diluting cell, strongly-ionized ions being removed first in the flowpath and weakly ionized species removed as the water moves down the flowpath. Removal of ionized species such as sodium, chloride, sulfate, and calcium by EDI is usually well over 99% and has been well documented previously [1][2][3][4]. Removal of weakly-ionized species is an area where a properly designed EDI can also achieve extremely high removal rates.
A number of operating EDI plants were closely reviewed for weak ion removal efficiency. In each case, EDI is an integral part of a multi-step process to produce ultrapure water, but the feedwater sources are quite different, subject to seasonal variations in quality, such as temperature, pH, TDS, and organic and suspended solids. The pretreatment processes and EDI are designed to handle these variations. Table 1 lists the processes that are used at each plant and shows some of the variables in each of the feedwaters.
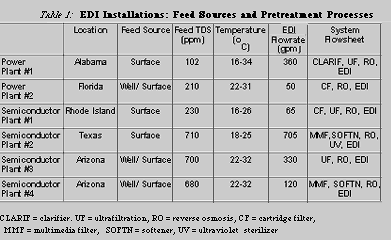
Silica Removal
Silica is one weakly-ionized ion that receives a lot of attention in water treatment for a number of good reasons. In power or boiler applications, silica scaling is a real and constant concern for plant operations. Failure to maintain low silica levels in ultrapure waters feeding these systems can result in damage to costly equipment such as heat exchangers or turbines. In addition, high silica levels in the boiler feedwater can reduce the amount of water cycling, directly reducing the efficiency of plant operations.
In semiconductor processing, high silica levels can lead to silica deposits on wafers which in turn lead to expensive chip failures. Silica levels are usually maintained at or below what can be detected by on-line instrumentation to prevent silica from affecting a semiconductor plant's wafer yield.
EDI is an excellent process for achieving low levels of silica. Typically, EDI removes over 98% of silica in a feedwater. Table 2 lists silica removal at six different operating plants. The data was obtained from both on-line and laboratory silica analyzers. The data shown is typical data for these EDI plants and demonstrates how EDI is able to achieve very low silica levels (less than 5 ppb), even when challenged with silica levels as high as 600 ppb or more.
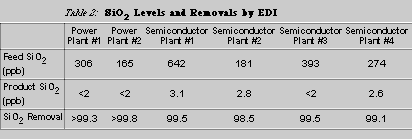
Silica Parametric Studies
To further explore the performance limits of EDI on weak ion removal, a laboratory study was conducted, focusing on the effect of flowrate and amperage on silica removal in a full-scale, 50 gpm unit. EDI feedwater for this test contained 20 ms/cm of NaCl and 0.5 ppm of CO2 when the feedwater was treated with RO.
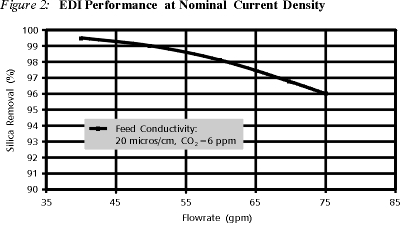
As shown in Figure 2, silica removal decreases with increasing flowrate for constant amperage. Increasing flowrate results in lower residence time, more utilization of available current for strong ion transfer, and less water splitting available for weak ion removal. However, even at velocities that exceed nominal velocities by 50%, silica removal remained above 95%.
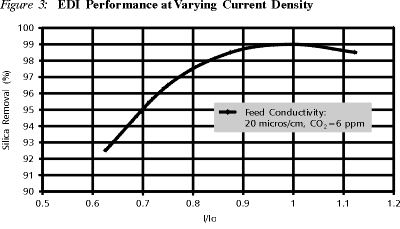
Figure 3 shows the effect of amperage on silica removal for a constant flow. Initially, silica removal increases with increasing amperage due to higher extent of resin/membrane regeneration; however, it levels off at a certain current I0 which is a function of flowrate and feed composition. Operation at higher current densities than I0 does not impact silica removal any further as the limiting factor at this point for silica removal is the residence time.
In another study, the effect of feed composition on silica removal was examined. Feedwater was prepared with RO permeate by chemical addition of mainly sodium chloride and sodium metasilicate. Feed conductivity varied from 20 to 200 ms/cm, and the amperage required to maintain silica removal values higher than 98.5% was found.
Figure 4 represents the results, showing that even at conductivities as high as 200 ms/cm, silica removal (>98.5%) can be obtained by appropriately increasing the current.
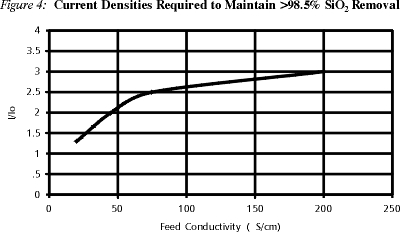
The above studies confirm that the degree of weak ion removal in an EDI unit is tied to the degree of water splitting occurring within it. The above studies also provide a basis for calculating design conditions for the removal of weakly-ionized species.
Carbon Dioxide
Carbon dioxide removal by EDI is an important facet of its performance. When carbon dioxide is present in an ion-exchange feedstream, the carbon dioxide competes with silica for ion-exchange sites on an anion resin. Carbon dioxide often represents the largest anion load on an ion-exchange system, especially when the ion-exchange unit is preceded by an RO unit. The presence of excess amounts of carbon dioxide both limits the capacity of ion-exchange resin to remove silica and limits the efficiency of silica removal by the ion-exchange bed. As discussed above, silica removal is critical in both power generation and in semiconductor production. The presence of an excess amount of carbon dioxide in effect exposes a plant to potentially serious silica problems. Carbon dioxide cannot normally be removed via other membrane demineralization processes such as RO and EDR unless chemical adjustments are made to change alkalinity levels. EDI, on the other hand, is a membrane demineralization process that routinely reduces CO2 levels by over 99% in most applications.
Table 3 summarizes the carbon dioxide removal at all six sites tested. Feed and product CO2 were in one case (semiconductor plant #1) directly measured using an on-line TOC/TIC analyzer. For the other cases, feed CO2 was determined using on-line pH and ion chromatography. The product CO2 levels were calculated using on-line instrumentation and ion chromatography. This method has been described previously and has been verified by laboratory and field testing [3].
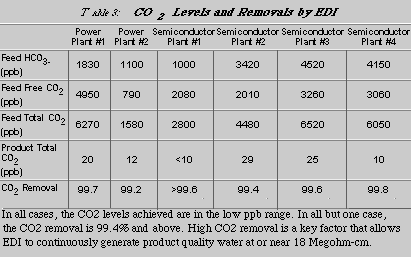
Figure 5 shows long-term product water quality from an EDI plant (semiconductor plant 1). This plant has been in operation continuously for over three years and, as shown, consistently produces water in the 17-18 Megohm-cm range.
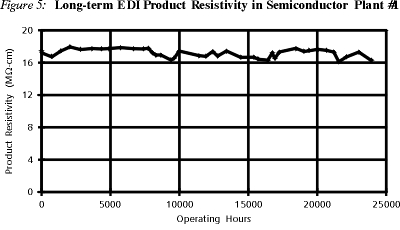
Repeated tests at this site have demonstrated that the only ion having any significant impact on EDI product resistivity levels is the carbon dioxide.
Boron
Boron is a species that has previously received little attention relative to other weakly-ionized species, such as silica and carbon dioxide. Boron is usually present in water supplies at very low concentrations, and typical water analyses do not even look for the presence of boron. Boron is often present in water though it is not always completely removed by many ultrapure water production processes. This is problematic as boron can cause defects in semi-conductor chip manufacturing [8].
In one well-documented case, feedwater boron of 130 ppb was causing operational problems at a semiconductor plant in Sunnyvale, California [9]. In this case, the demineralization process consisted of RO followed by a conventional ion-exchange system. Although the RO was doing a very good job at ion removal, including silica removal, it was not doing a good job at removing boron (59%). This is a fairly common boron rejection level when using thin-film composite RO membranes. Boron was not being well removed by the ion-exchange process due to its poor ionization and low selectivity [8], which causes boron to break through much faster than silica. This allowed high levels of boron (30 ppb) to enter the semiconductor plant polishing loop water. In this case, the solution was to routinely monitor the ion-exchange effluent for boron and to reduce the throughput of the ion-exchange system by approximately 50% from what was anticipated, which added cost.
This is not an isolated case. The authors are aware of other ultrapure water plants where boron has made its way into the final product water entering the fab or processing facility. In many cases, ultrapure water systems accomplish demineralization either by RO or double-pass RO. Although RO is excellent for most ions, it is a relatively poor performer when it comes to boron. Conversely, EDI is an excellent boron remover.
Table 4 shows removal of boron by both RO and EDI at four plants. The data was obtained via grab samples which were analyzed using ICP-MS with a detection limit of 0.05 ppb. As shown in the table, the RO units typically remove only a small portion of the boron from the feedwater. However, the EDI units consistently remove over 96% of the boron in the feedwater. The data also shows that EDI is much better at removing boron even though the pH of the EDI feedwater is less than that of the RO feedwater. Because EDI ionizes the weakly-ionized boron, it can remove this species to such a high degree.
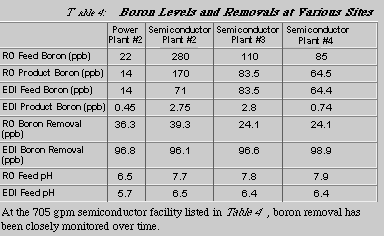
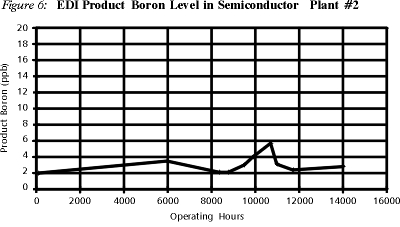
At the 705 gpm semiconductor facility listed in Table 4, boron removal has been closely monitored over time.
As can be seen in Figure 6, the level of boron in the EDI product water has remained consistently low over a period of 14,000 hours of operation, corresponding to EDI's boron removal rate of over 96% on a consistent basis. High boron removal is one of the many reasons that EDI use has been rapidly growing in the semiconductor field.
Ammonia
An extensive study on ammonia removal by EDI was performed by Eltron Research, Inc. on behalf of NASA. The EDI unit utilized in this study was provided by Ionics. Ionics worked closely with Eltron [10] over the course of this project.
The ammonia study was part of a larger project addressing the development of total water-recycle systems for Space Station. In a self-contained life support system, such as in the future-planned Space Station, the ability to efficiently recycle water is key to long-term mission success. It is projected that a four-person team will use a supply of 225 lbs of water for general use during a space mission to Mars and back. All water used will need to be recycled through an intricate recovery system and treated through a sequence of processes to produce drinking water. Ammonium ions are generated as a decomposition product of urea and over time will build up in concentration, thus rendering the water unsuitable for human consumption. EDI, being one of the purification steps, is required to reduce the level of ammonia from 200 ppm down to the range of 0.5-1 ppm.
For the pilot runs at Eltron, the EDI synthetic feedwater contained 200 ppm ammonia at a pH of 10.3. Such a pH, well above the ammonia pKa of 9.25, means that the ammonia solution is largely in non-dissociated form. Eltron Research, Inc. [10] has provided us with some of their results from a long term study they performed.
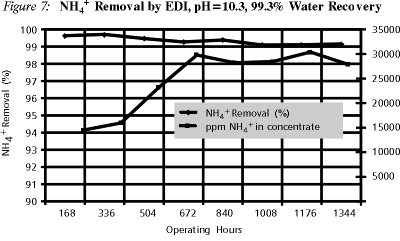
Figure 7 shows the percent ammonia removal by EDI. EDI operation was optimized in terms of flowrate and current. In earlier experiments it was found that operation at current efficiencies in the range of 20-30% was necessary to maintain the resin in regenerated form and thus obtain high ammonia removal.
The system was operated in a semi-continuous mode with a continuous 200 ppm ammonia feed at 160 ml/min. The concentrate stream in turn was continuously recycled from a separate waste tank. The waste tank volume was kept constant by periodically removing a small amount of water that would make up for any water transfer from the dilute to the concentrate through the membranes. The amount of periodic waste blowdown corresponds to a water recovery value of 99.3%. Due to such continuous recycle, the waste concentration increased over time as pictured in Figure 7. Such high concentration resulted in turn to a slight decrease in ammonia removal due to back-diffusion.
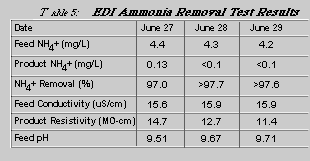
Another in-house study on ammonia removal by EDI also resulted in ammonia removal levels of >98% (Table 5). Due to the ammonia content of the EDI product reaching the detection limit of the analytical method used, we could not really observe any higher removal values.
Conclusion
EDI usage has grown considerably in the last few years because of its ability to remove weakly-ionized species. Compared to other membrane processes such as RO, the performance of EDI on weakly-ionized species is clearly superior.
The performance that EDI has demonstrated in removing difficult ions, such as silica and carbon dioxide, is reason enough to expect the continued future growth of EDI. As quality standards for ultrapure water continue to evolve, the presence of such ions as boron at very low levels will become a more important issue. EDI's performance in removing these weakly-ionized species will thus assuredly lead to greater use of EDI in the production of ultrapure water.
As new challenges arise in the field of water and wastewater treatment, new applications, taking advantage of the EDI's ability to remove weakly-ionized species, will undoubtedly add to the usage of EDI in the future.
References
- Hernon, B.P.; Zhang, Li; Siwak, L.R. and Schoepke, E.J., "Progress Report: Application of Electrodeionization in Ultrapure Water Production," International Water Conference, Pittsburgh, PA, October 29-November 2, 1995.
- Hernon, B.P.; Zanapalidou, R.H.; Zhang, L.; Siwak, L.R. and Schoepke, E.J., "Application of Electrodeionization in Ultrapure Water Production: Performance and Theory," International Water Conference, Pittsburgh, PA, October 30-November 2, 1994.
- Hernon, B.P.; Zanapalidou, R.H.; Zhang, L.; Sims, K.J. and Siwak, L.R., "Electrodeionization in Power Plant Applications," Ultrapure Water, July/August, 1994, p. 33.
- Smith, G.O. and Wilson, K.S., "Makeup Water Treatment Utilizing Triple Membrane Demineralizers at Entergy Operations, Inc.'s Grand Gulf Nuclear Station," Ultrapure Water Expo '90 West, Conference on High Purity Water, San Jose, CA, November 28-29, 1990.
- Comb, L. and Schneekloth, P., "High Purity Water Using Two-Pass Reverse Osmosis," Ultrapure Water, April, 1990, p. 49.
- Pittner, G.A.; et. al., "Unique Double-Pass Reverse Osmosis System Eliminates the Need for Many Deionization Applications," Ultrapure Water, September/October, 1986, p. 23.
- Lesan, R.; et. al., "A Comparison of Different Classes of Spiral-Wound Membrane Elements at Low Concentration Feeds," Ultrapure Water, April, 1990, p. 18.
- Yagi, Y.; Hayashi, F. and Uchitomi, Y., "Evaluation of Boron Behavior in Ultrapure Water Manufacturing System," Proceedings of the Semiconductor Pure Water and Chemical Conference, San Jose, CA, March 8-10, 1994, pp. 54-62.
- Malhorta, S.; Chan, O.; Chu, T. and Fucsko, A., "Correlation of Boron Breakthrough versus Resistivity and Dissolved Silica in RO/DI System," Ultrapure Water, May/June, 1996, p. 22.
- Spiegel, E.F., "Personal Communication," Eltron Research Inc., Boulder, CO.
