Effective Lay Up/Start Up of Utility Steam Generators Using Low Residual Inhibitors
By Kaj D. Rondum, Douglas B. DeWitt-Dick, Ashland Specialty Chemical Company Drew Industrial Division, and Ron L. Melton, Western Farmers Electric Cooperative
Introduction—Traditional Procedures Challenged by a New Way
Excess generating capacity forced many utilities to convert base loaded units to cycling units. The factors contributing to this reserve of spinning capacity relate to faulty load demand forecasting, a relocation of heavy manufacturing facilities and the introduction of new generating capacities. While all of this may be temporary, it still places the burden of protecting the idle equipment on the shoulders of plant personnel.
To have these units available for rapid dispatch, they are generally laid up wet. Traditional wet lay-up procedures use high levels of a volatile oxygen scavenger/metal passivator along with ammonia for pH elevation, topped off with a nitrogen blanket. While this procedure provided good protection of the steam generator during lay-up, it created problems with elevated levels of ammonia and/or cation conductivity during start up.
In all-steel systems, the elevated ammonia levels weren't as big a concern as they were in mixed metallurgy systems; however, high cation conductivity should be a concern in all systems. This left the plant with the choice of either trying to combat the water chemistry problems or dumping the unit and refilling it with fresh water. In either case, "crud" transport was a problem, and the time from initial start to generator roll could be quite lengthy.
A new procedure was developed to eliminate the high levels of oxygen scavenger/metal passivator required for protection during lay-up, to stabilize water chemistry and minimize crud transport during start-up and to reduce the time it takes to bring a stand-by unit into service.
This paper discusses the procedure, outlines the modifications necessary to implement the program and documents the results achieved to date by comparing several start-ups of a high pressure (1800 psig) cycling unit using this method with a similar unit at the same station using the traditional lay-up/start-up procedures. The authors hope to present the utility industry with a viable method of protecting idle steam generators, extending the life cycle of the unit, and improving the economics of bringing a unit back into service.
Plant Description
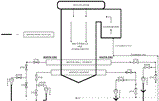
This unique procedure using a circulating mode was implemented in Unit 2 at Western Farmers Electric Cooperative in Mooreland, Oklahoma. This utility operates three units. Units 2 and 3 operate at 1800 psig, 1005°F superheat/reheat with rated capacity of 125 MW and 135 MW respectively. Unit 1, which operates infrequently, operates at 1250 psig, 950°F superheat with a rated capacity of 44 MW.
Because of the current environment within the power generation industry, this plant and many others have been forced to operate in a cycling mode. This plant has operated in this manner since the early 1980s as the economics of cycling are well established in the industry.
While cycling operation is a viable alternative to peaking, certain considerations need to be made relative to system chemistry. A schematic diagram of Unit 2 is shown in Figure 1.
Conventional Lay Up/Start Up
All three units at this plant have traditionally been put into wet storage using 200 ppm of hydrazine and ammonia as required. After coming off line, the boilers were allowed to cool and the chemical treatment solutions were added. The boilers were then put on low fire to provide adequate circulation of the treated water and to enhance the oxygen scavenging and metal passivation reactions through thermal catalysis.
While this procedure is acceptable and has been used by many utilities for lay up, the start up phase can create some difficulties, particularly with respect to water chemistry and water management. To avoid excessive ammonia levels at start up the boiler was completely drained of its 20,000-gallon capacity. If the unit wasn't drained of the relatively high level of hydrazine, the plant experienced ammonia levels of 2000–3000 ppb in the steam following start up.
This required excessive blowdown to reach desired chemical control levels. Since the treated water contained 200 ppm of hydrazine, environmental concerns relative to discharge requirements existed as did worker safety concerns associated with handling hydrazine solutions. The boiler then needed to be refilled with demineralized makeup water from the condensate storage tanks.
The plant is strictly limited on demineralized water output (100 gpm) and storage. Four, 30,000-gallon condensate storage tanks can provide the capacity to refill the boilers, but not without reducing the safety and assurance buffer that they provide should the boiler suffer a leak. Furthermore, increased demineralizer throughput reduces the life expectancy and performance of ion exchange units, particularly with respect to the anion beds. This can result in increased organic loading, fouling, and sloughage leading to organic acid formation.
Additionally, the increase in regeneration manpower requirements, chemical costs and regenerant waste disposal further exacerbate the economic situation. Since the condensate storage tanks are vented to atmosphere, the water is fully oxygenated and chemical demand requirements are high, generally about five times the "typical" hydrazine dosage.
Depending on the amount of time the unit has been out of service, repassivation reaction can consume all of the hydrazine residual. To compensate, the plant would have to continually increase the hydrazine feed rate while suffering the high pH effects in the steam due to excess ammonia generation. Often times, a hydrazine residual could not be achieved, and the plant subsequently increased ammonia feed to maintain steam pH levels, and it might take up to three days for conditions to stabilize.
During the start-up phase, the system water chemistry characteristics including silica, ammonia, pH, hydrazine, phosphate, conductivity and cation conductivity require increased attention from the station chemists until a steady state condition is reached. Because of the extensive repassivation that occurs, iron oxide sloughage can raise boiler water particulate iron levels above 1000 ppb, sometimes reaching 5000 ppb.
The plant, therefore, holds the unit at 1500 psig while continuing blowdown until the iron level drops below 200 ppb. The unit is then released for full pressure (1800 psig and 45 MW), a complete set of water analyses is run, and the unit is released to dispatch.
The entire procedure including draining, refilling and firing the boiler for start up typically requires 12 hours. Recognizing the operational constraints, time factors, economic impact, as well as environmental and safety concerns, the plant deemed it necessary to evaluate an alternative lay-up procedure.
Improved Lay Up/Start Up Method—Meet MEKO
While hydrazine has been used successfully in the utility industry for more than 40 years, recent advancements in the use of hydrazine alternatives for improved corrosion inhibition have drawn attention in the marketplace.
Methyl ethyl ketoxime (MEKO) is a unique reactive oxime-based oxygen scavenger/metal passivator, which has been used as a superior alternative to hydrazine and other organic hydrazine alternatives in more than 60 utility units in North America. Based upon its physical characteristics, low toxicity, performance history in the utility sector and well-established track record, MEKO was selected for this unique lay-up scenario.
Because Unit #2 operates most efficiently and therefore cycles most frequently, it was selected for a minor retrofit modification, which involves a boiler lay up re-circulating system. Since MEKO was used in place of hydrazine for treatment in the new re-circulating loop, the existing chemical feed system was used.
This required the addition of a heater, back pressure and bypass valves and a small amount of associated piping. This is because MEKO requires thermal activation to form the intermediate reactive species, which remains active even at ambient conditions encountered during lay up.
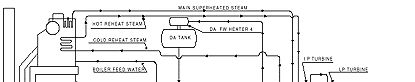
The temperatures found in the feedwater circuit of on-line steam generating systems are more than adequate to form the reactive species and hence a separate heater isn't required for operating systems. The chemical feed system is designed so that a MEKO solution can be introduced either into the condensate pump discharge header or the economizer inlet header, the latter of which is employed. A schematic flow diagram of the system is shown in Figure 2.
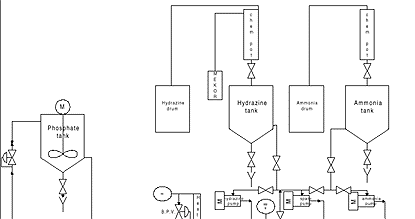
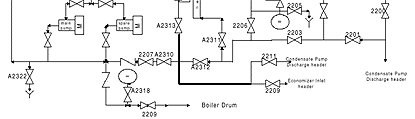
While procedures exist for traditional lay up using MEKO, including firing of the boiler to thermally catalyze reactions and provide re-circulation, the incorporation of an external heater and a re-circulating pumping system offers distinct advantages, especially in comparison to hydrazine.
The boiler re-circulating system for lay up using MEKO required some minor piping, valving and hose connections. The boiler drains from four locations, which are the north and south water wall and hopper headers. Each of these required an additional high-pressure isolation valve and a quick disconnect and flexible hose.
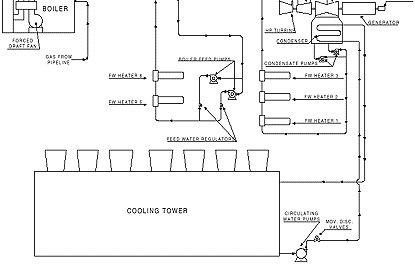
This ties into a common suction header to the 5 hp boiler re-circulating pump, which discharges to the high-pressure feedwater heaters prior to the economizer and into the boiler completing the loop. A schematic flow diagram is shown in Figure 3.


Results—No Draining Required
The MEKO goes into the system for lay up at about 5 ppm, which in this case is about one pint. Because of the relatively low level required for system protection, the boiler doesn't have to be drained, saving the plant 20,000 gallons of water each time and avoiding the discharge of 20,000 gallons of hydrazine-containing water.
From a chemical usage perspective, the plant previously used about 10 gallons of hydrazine, which would then be disposed of along with 20,000 gallons of water. With MEKO, the usage is about one pint and is only supplemented when required as indicated by chemical residual testing.
The chemical cost savings with MEKO are therefore two fold in that the chemical residual required is 5 ppm versus 200 ppm, and the system residual isn't dumped and therefore doesn't require replenishment. With respect to demineralized water management, the ion exchange unit throughput is dramatically reduced, extending life expectancy and minimizing wear and tear on the units.
Also, re-generant chemical usage and associated waste streams are minimized, and the condensate storage tanks are able to remain closer to capacity. Using the re-circulating loop allows for maximum chemical contact with metal surfaces and eliminates stagnant situations. It also allows the system to remain full of treatment chemicals at levels that remain much closer to normal operating conditions.
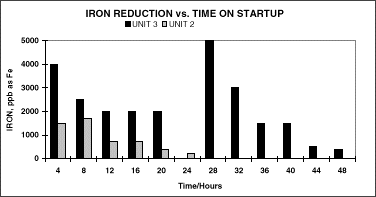
This in turn dramatically reduces the time to reach proper chemical control parameters and subsequent release of the unit to dispatch. In addition, MEKO forms an extremely strong and durable passivated oxide film on metal surfaces, thereby reducing iron throw. Iron levels upon start up after using the re-circulating loop with MEKO average about 500 ppb initially but dropped below 200 ppb almost immediately.
Since most, if not all, other water chemistry parameters typically remained within specified control ranges, iron tends to be the limiting factor in releasing a unit to full pressure. Since the unit can go up in pressure faster, it operates at a better heat rate and is consequently more efficient. This results in more rapid release for dispatch and unit availability and reduces the time to start up a unit by more than two hours (~15%).
Conclusion
A novel method has now been developed and proven effective in laying up cycling units. This procedure reduces the time required to bring the unit online, the amount of demineralized water used, the potential discharge of regulated chemicals and the amount of crud transported to the boiler.
Further investigations, which weren't included as part of this paper indicate significant reductions in deposit weight densities using this method of lay up for cycling units.
The Mooreland plant considers the benefits and results obtained from Unit #2 to provide sufficient justification to warrant implementation of the procedure for Units #1 and #3. Currently the installation of the system for Unit #3 is complete with the exception of a written operating procedure. Cost for the re-circulating lay up system for Unit #1 was estimated at US$15,000 in 1998 for this size unit.
Savings associated with the time reduction between start up and availability for full-load dispatch could potentially reach a maximum of US$7,000 per start up and doesn't include the additional savings outlined previously. The authors consider this to be a viable, cost-effective alternative to traditional lay up methods for cycling units.
A comparison of iron levels and time to reach compliance levels for Unit #2 and #3, which use MEKO and hydrazine, respectively is shown in Figure 4.
Acknowledgements
The authors wish to express sincere appreciation to the staff at Western Farmers Electric Cooperative's Mooreland plant for its support without which this paper wouldn't be possible.
For more information about MEKO and its success in the field or to discuss this particular project with one of the authors, contact Ashland Chemical Company, Drew Industrial Division, Anne Cantafio, arcantafio@ashland.com, 973-263-7745.
Edited by April C. Murelio
editor@poweronline.com
